Targets
Modern drug discovery is aimed at an increasing variety of targets.
At Excellerate we have extensive experience working with a wide range of drug target families, including G protein-coupled receptors (GPCRs), kinases, nuclear receptors, enzymes and E3 ligases (targeted protein degradation).
We also have expertise in developing bespoke assays for non-standard target classes, such as subcellular organelles.
Our Capabilities
Targets
Target Class
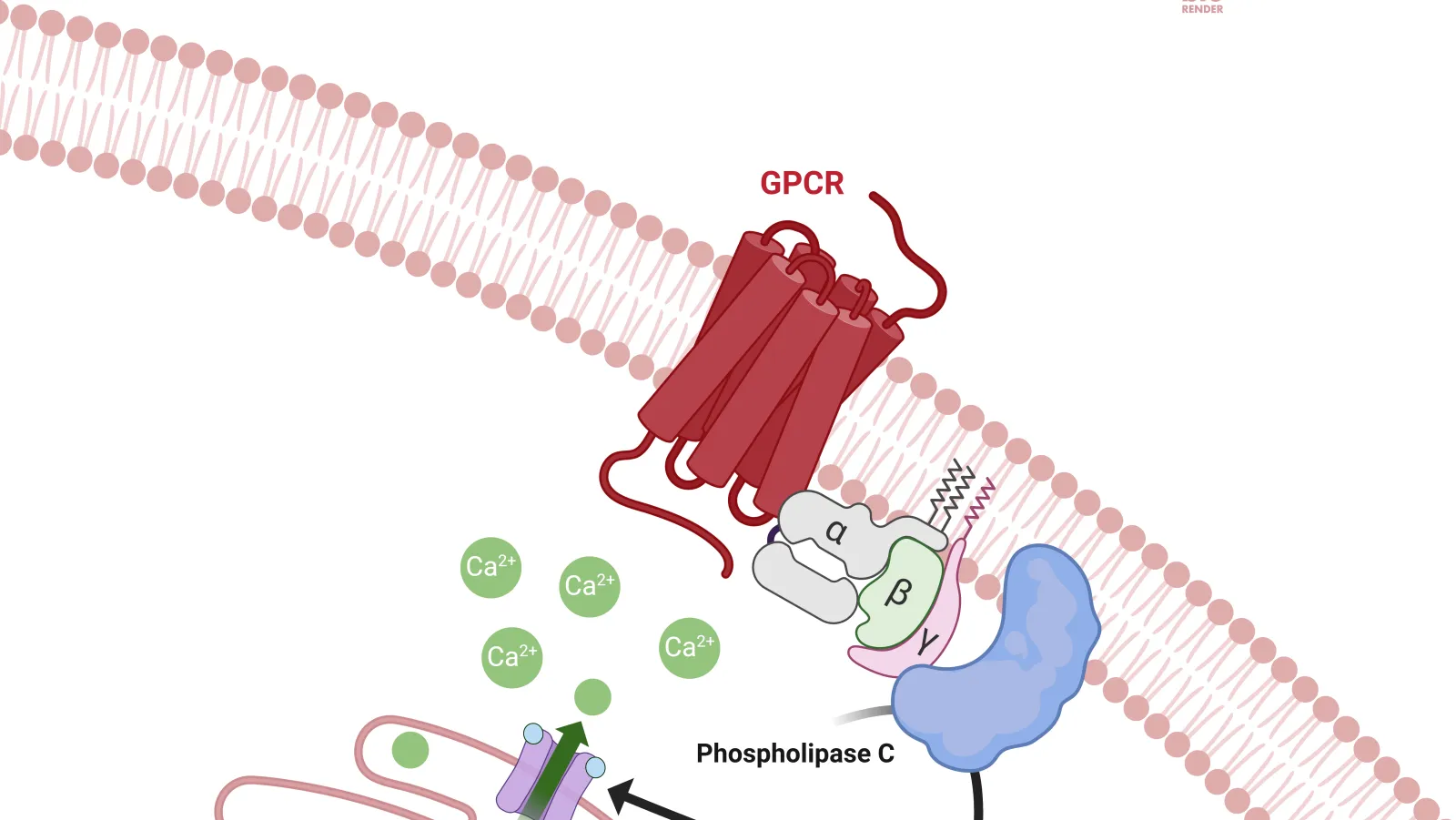
GPCRs
G protein-coupled receptors (GPCRs) are a major superfamily of over 800 cell surface receptors responsible for many physiological and pathophysiological processes.
They continue to be a major target for drug development and de-orphanisation including a range of pharmacological modalities such as biased ligands, allosteric modulators and modulatory antibodies.
Excellerate provide a full range of assays for characterizing GPCRs and quantifying their pharmacology including:
Receptor Binding: Established and bespoke membrane and whole cell competition binding assays to determine ligand binding properties to GPCRs including affinity, binding kinetics, allosterism and SAR of a compound series.
Downstream Signaling: Downstream signaling responses and compound efficacies using multiple signaling outcomes e.g. live cell biosensor assays, including G protein activation, cAMP levels, inositol phosphate production, intracellular calcium release and ERK activation.
Desensitization and Internalization: GPCR interactions with β-arrestin, imaging of cell surface and intracellular GPCR localization, quantification of cell surface GPCRs using ELISA.
Phenotypic Responses: whole cell responses stimulated by activation of endogenous and exogenously expressed GPCRs including proliferation, differentiation, and metabolic changes in a range of cell types.
Kinases
Kinases are a large family of enzymes which phosphorylate serine, threonine or tyrosine residues to regulate activity and signaling.
They include both cytosolic enzymes and cell surface receptor tyrosine kinases (RTKs), predominantly for growth factors, which have intrinsic tyrosine kinase activity in their intracellular domain. They are drug targets for a range of proliferative and immunological conditions through a range of drug modalities including biologics and small molecule kinase inhibitors.
Excellerate has extensive experience working with kinases and RTK targets, and provide a full range of assays for the pharmacological characterization of drugs acting at kinases including:
Receptor Binding: Established and bespoke membrane and whole cell competition binding assays to determine ligand binding properties to RTKs including affinity, binding kinetics, allosterism and SAR of a compound series.
Downstream Signaling: Assays for receptor activity such as receptor autophosphorylation and tyrosine phosphorylation of signaling components, ERK activity and activation of transcriptional activity.
Phenotypic Responses: whole cell responses stimulated by activation of endogenous and exogenously expressed RTKs, or involving cytosolic kinases, including proliferation, differentiation, and metabolic changes in a range of cell types.
Nuclear Receptors
Nuclear receptors are a family of intracellular receptors which are transcription factors activated by a range of lipophilic ligands such as steroid hormones and lipids.
They are involved in a range of homeostatic and metabolic functions throughout the body and are targeted in a range of diseases, including inflammation, asthma, fertility and metabolic disorders.
Excellerate provides multiple assays to monitor nuclear receptor interaction and activation:
Receptor Translocation: Image-based assays to monitor nuclear receptor and transcription factor location in cytosol and nucleus
Transcription Factor Activation: Cell-based reporter gene assays for transcription factor activation including those working through CRE, NFAT and SRE.
Phenotypic Responses: Whole cell responses stimulated by activation of endogenous and exogenously expressed GPCRs including proliferation, differentiation, and metabolic changes in a range of cell types.
Target Class
Targeted Protein Degradation
The ubiquitin-proteasome system is fundamental to cellular homeostasis, regulating protein turnover through precise degradation mechanisms.
The E3 ubiquitin ligase family, which facilitates the transfer of ubiquitin to specific protein substrates, has emerged as a key focus in drug discovery, enabling novel therapeutic strategies such as PROTACs and molecular glues.
At Excellerate, we have developed specialized assays to assess the effects of candidate compounds on targeted protein degradation:
Targeted Protein Degradation (TPD) Assays: Cell-based immunofluorescence, Western blot and NanoBRET approaches to quantify protein degradation with high sensitivity. In addition, we can assess subcellular specificity of degradation (e.g., nuclear degradation).
PROTAC and Molecular Glues Profiling: Characterization of time-course kinetics and E3 ligase selectivity to determine compound potency and specificity. Assays include ternary complex formation studies and target ubiquitination efficiency.
Enzymes
Understanding the reaction mechanisms of enzymes and the molecular mode of action of enzyme inhibitors is essential for the discovery and development of potent, safe, and effective drugs.
At Excellerate, we leverage detailed mechanistic enzyme kinetics to characterize enzyme targets, profile compound properties, and gain insights into the mechanisms of action required for drug efficacy.
We offer a comprehensive range of enzyme assays, including:
- Enzyme Activity: Quantitative measurement using fluorometric, colorimetric, and luminescent readouts for detailed analysis of compound efficacy and potency.
- Enzyme Kinetics: Full Michaelis-Menten analysis and mechanism of inhibition studies, providing insights into binding affinities and modes of action.
- Substrate Profiling: Screening of substrate specificity and turnover rates to characterize enzyme-substrate interactions and identify alternative substrates.
- Hit Identification and Validation: Extensive use of enzyme assays in hit identification, validation, and detailed characterization of compound mechanisms to guide lead optimization.
- Mechanism of Action Studies: Advanced approaches to probe the relationship between molecular structures and binding kinetics, inhibition, and mechanisms of action, moving beyond traditional IC50 values.
- Biophysical Integration: Combining enzyme kinetic studies with biophysical methods to assess protein quality, identify desired mechanisms, and enhance mechanistic characterization.
Non-Standard Targets
Advances in genomics, bioinformatics and our understanding of cell biology means that the range of drug targets is continually expanding and requires and equally fast-moving approach to developing new assay modalities.
We have a breadth of experience across pharmacological areas which allows us to respond quickly and develop new approaches to quantifying drug-target interactions in new areas.
Cytokines/Growth Factors: Assays available for immunomodulation assessment, cytokine release and ELISAs.
Subcellular Organelle Proteins: Organelle isolation, assays for lysosomal activity and mitochondrial function.
Protein-Protein Interactions: A range of FRET, BRET and complementation assays are available to detect PPIs and the effects of candidate drugs.
Cell Cycle, Apoptosis & Autophagy: Cell cycle progression and arrest, apoptotic pathway activation (caspase activity, annexin V), and autophagy modulation.


